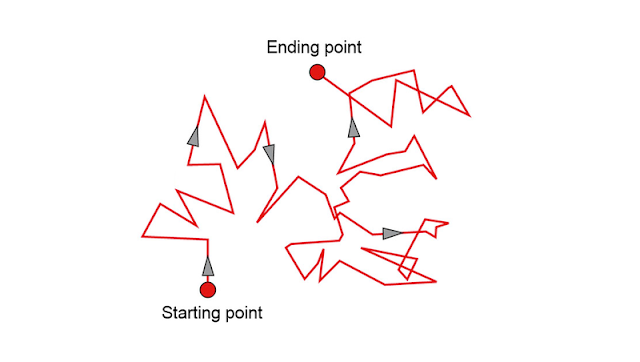
Brownian motion occur due to the bombardments of the particles in colloids. The bombardment by dispersion medium is obviously unbalanced. Thus zig-zag motion like the above figure is noticed.
The phenomenon was first observed by Robert Brown. The motion depends on size of the particle and viscosity of the fluid.
Smaller the size of the particle, faster the motion. Because of momentum transfer.
Higher the viscosity lower the motion of the particle. As it is inversely proportional to it. If you have any question or, opinion, follow the comment box below.
Photo Credit: Nived Rajeev
This Photo is licensed under the Creative Commons Attribution-Share Alike 4.0 International license.

No comments:
Post a Comment